Salt batteries don't burn!
«Explosion in residential house in Bodnegg – battery storage of photovoltaic system detonated.»
www.all-in.de (German only)
«Manufacturing company draws consequences after explosion of battery storage unit.»
www.swr.de (German only)
«Highly toxic acid leaked in fire from electricity storage unit.»
www.rbb24.de (German only)
«Hyundai replaces batteries in 80,000 electric cars due to fire hazard.»
www.fokus.de (German only)
Headlines about burning batteries make the rounds again and again. From smartphones in your pocket to drive batteries in e-vehicles to home storage in the basement, the topic of fire hazards and fire safety is widely discussed. It is undisputed that lithium-ion batteries are fire accelerants. At the end of the following article, you will find out how this works in detail, what needs to be done and what experts think about it. And, to say it in advance: All these fire horror stories do NOT apply to salt batteries. These are absolutely safe!
SALT BATTERIES
Hot and yet not flammable
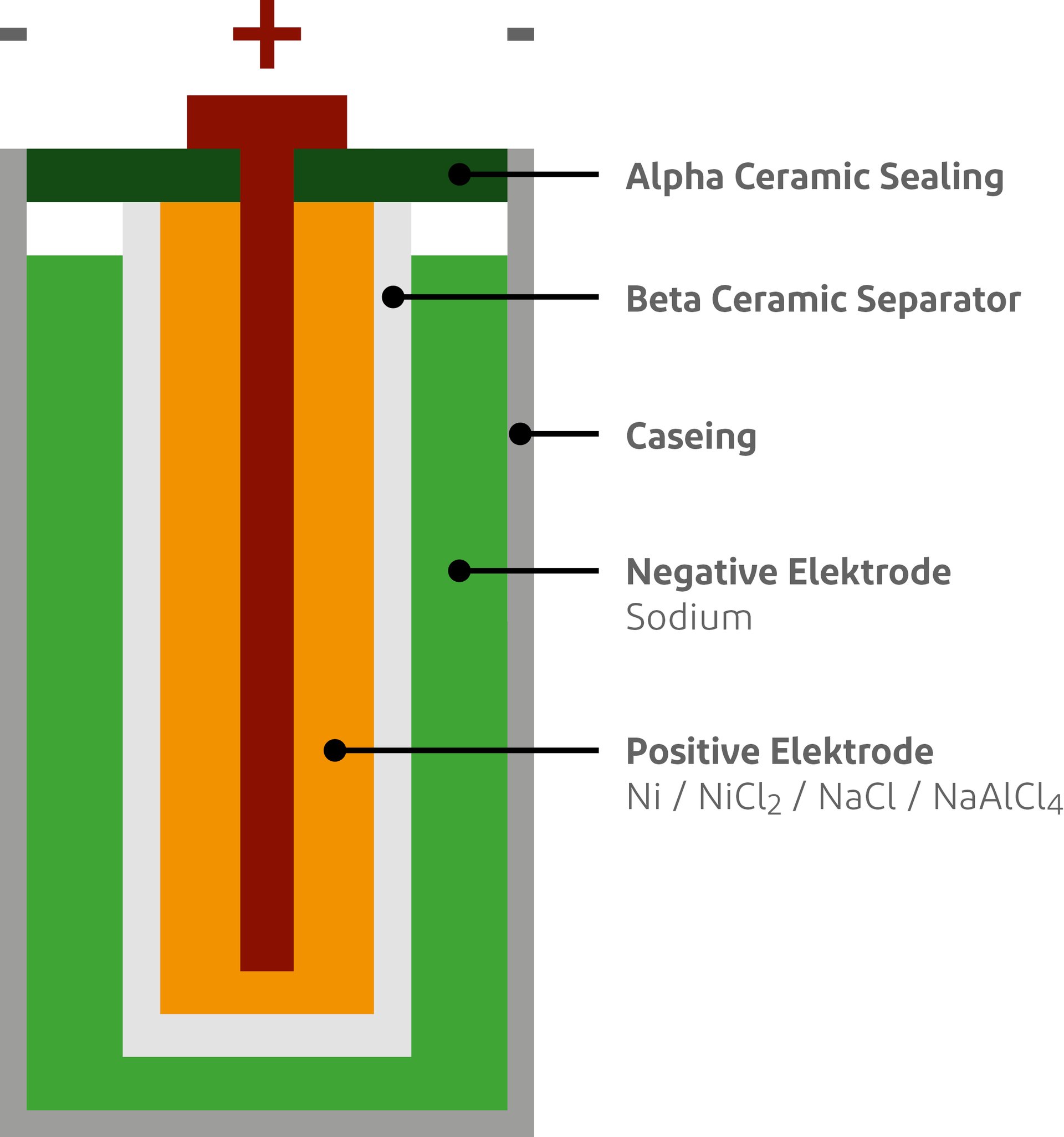
As the name of the battery suggests, the salt battery consists mainly of common salt. Every living being needs salt and we consume it every day with our food. It is therefore a harmless raw material that is also available in sufficient quantities worldwide. The active mass of the salt battery consists of common salt and nickel. The electrolyte consists of common salt with a small pinch of aluminium and becomes liquid at 150° Celsius. At a temperature of 250 degrees, the nickel-salt mixture can be used to store energy.
Now, one might assume that a hot battery also burns much faster. But that is not the case at all. It is rather the other way round: because this battery works at high temperatures, it is less sensitive to outside temperatures. And: Crash and gunshot tests in combination with water have clearly shown that this high-temperature battery neither explodes nor burns.
Behaviour of a salt battery in case of damage
It does not matter whether a salt cell is damaged by fire or by any other external influence. The behaviour is always the same. So what happens in the event of damage to one or more salt battery cells?
The steel casing of a cell is deformable and bulges inwards when the outer casing is subjected to pressure. It does not matter whether the steel wall is only dented or destroyed. The liquid sodium metal on the outside in the charged state passes this pressure on to the beta ceramic separator on the inside. This causes the brittle and thin-walled ceramic to shatter. The two hot substances run into each other and react within fractions of a second to form sodium chloride (common salt) and aluminium. During this reaction, the temperature inside the battery rises from 250° Celsius to 450° Celsius for a brief moment and then drops to ambient temperature. Both materials are solid after cooling, so nothing can leak out.
Inorganic electrolytes, as in the case of the salt battery, do not contain carbon. They are salts and metals that can neither outgas nor burn. Try holding a lighter under a salt shaker. Salt batteries are not fire accelerants. The fire brigade is told at this point that a salt battery does not require any safeguarding or fire prevention measures.
MIGROS and the building permit authority
When the MIGROS large storage tank with 540 kWh was installed, MIGROS proudly published several articles about this currently largest salt battery storage in Switzerland. An employee of the building commission Schlieren/ZH read about it and sounded the alarm. In the case of large storage facilities, especially in public areas, they must be secured by structural measures in accordance with regulations for fire and personal protection. Such a building permit was not available. He ordered the immediate shutdown of the storage facility. In an emergency meeting with the authorities, MIGROS and innovenergy, the technology was explained in detail. Within an hour, the issue was off the table and MIGROS' large-scale storage facility was allowed to go back online. There was no danger whatsoever from this storage facility and no structural measures were necessary. To this day, this storage unit is running perfectly.
LITHIUM-ION BATTERIES
Causes and fire hazards of lithium-ion batteries
The risk of explosion with lithium-ion batteries varies and is very much due to storage and external influences. A dropped smartphone can cause internal short circuits and ignite the battery. Overcharging lithium-ion batteries can also cause internal damage. Large external thermal impacts, for example due to solar radiation, are possible causes of spontaneous combustion. Therefore, lithium-ion batteries (LIB) should always be stored and operated within the prescribed temperatures. As a rule, this temperature range is between 5° and 25° Celsius.
In the event of a fire approaching the battery from the outside, LIBs are considered fire accelerants, which is their real danger. «Even when already relatively small quantities of lithium-ion storage media are stored and catch fire, a very rapid spread of fire is to be expected. Burning parts can be thrown around due to the sometimes explosive burning,» says Christian Emrich of the Munich Fire Brigade.
Problems in case of fire with lithium-ion batteries
Another danger is that toxic gases escape in the event of a fire. It is therefore advisable to move away from the site of the fire quickly. Firefighters know that LIBs cannot be extinguished; they can only be cooled with a lot of water. «Smothering by means of pillows, sand, CO2 or other extinguishing agents does not work because the chemical processes have to be cooled. The oxygen that the combustion process needs is already chemically bound in the cells. Therefore, 'covering/smothering' the source of the fire does not help,» explains the Munich Fire Brigade. In the aftermath of a LIB fire, the «burnt-out» battery must be placed under thermal observation in a water basin, as reignition cannot be ruled out. Several days can pass between the time when the fire is «under control» and the time when the fire is «out».
batteries in cars, the question arises again and again to what extent an e-vehicle is more likely to catch fire than a vehicle with a combustion engine. Here, the opinions of researchers, fire brigades and vehicle testers largely coincide. «To date, several real fire tests have been carried out on passenger cars, which showed that the fire behaviour of e-vehicles does not differ significantly from that of conventional vehicles. However, the smoke composition is noticeably different due to the increased occurrence of hydrogen fluoride and phosphoric acid, both of which pose an increased health risk,» is the statement of an Austrian research group.
Experiments by fire brigades have also shown that the fire intensity does not depend on the type of drive, but is determined by the materials installed in the vehicle, i.e. essentially by the interior of the vehicle. As a rule, the fire does not come from the drive technology, but from the outside. Vehicle batteries are very well sealed off. Only in the worst case, when the protective devices of the drive batteries have been impaired in serious accidents, does the feared «thermal runaway» occur. This means that the temperature rises within a very short time due to an unstoppable chemical chain reaction and abruptly releases the energy stored in the battery. This process is equivalent to an explosion.
Besides the toxic gases during a battery fire, the subsequent soot deposits in the vicinity of the fire are very dangerous. They contain large amounts of cobalt oxide, nickel oxide and manganese oxide. These heavy metals are corrosive, allergenic and carcinogenic. Likewise, the extinguishing water is a toxic mixture that should not enter the normal sewage system, let alone the surrounding nature.
Second-life batteries
What has not been thoroughly researched so far is the behaviour and hazards of end-of-life lithium-ion batteries. Not much is known yet about the use of ageing or second-life energy storage devices, which are often used in buildings or at charging stations after use in e-vehicles. There is an urgent need to catch up here.