Where does it make sense to use a salt battery?
- Everywhere where a lot of energy is produced and medium power is required.
- Everywhere where a long service life is required.
- Everywhere where maintenance-free use is necessary.
- Everywhere where minimising the fire load is essential in critical infrastructure systems.
- Everywhere where extreme climatic conditions prevail.
In concrete terms, this means households and commercial enterprises that require a stationary battery storage system for normal medium power demands.
What is the salt battery not suitable for?
Anywhere where high power is required, such as eMobility or grid services.

Advantages of the salt battery
Salt batteries ...
- are made of harmless materials.
- Battery efficiency 90 % in standard cycle.
- are 100 % recycled.
- promote Swiss and European value chains.
- are absolutely safe (non-flammable, non-explosive).
- require no additional construction measures (no fire protection, no temperature control, no ventilation).
- can be installed almost anywhere - it has to be dry (cellar, garage, shed, attic, etc.).
- run even under extreme temperatures from -20° to +60° C.
- can be completely deep-discharged or put into hibernation.
- have a service life of at least 15 years and are maintenance-free.
- are extremely robust and have a high energy density (like LIB).
Disadvantages of the salt battery
Salt batteries ...
- are not performance batteries for high charge and discharge currents.
- require some internal energy to maintain the working temperature.
- are high-priced because they are not produced in low-wage countries and do not enjoy the economies of scale of high production volumes.
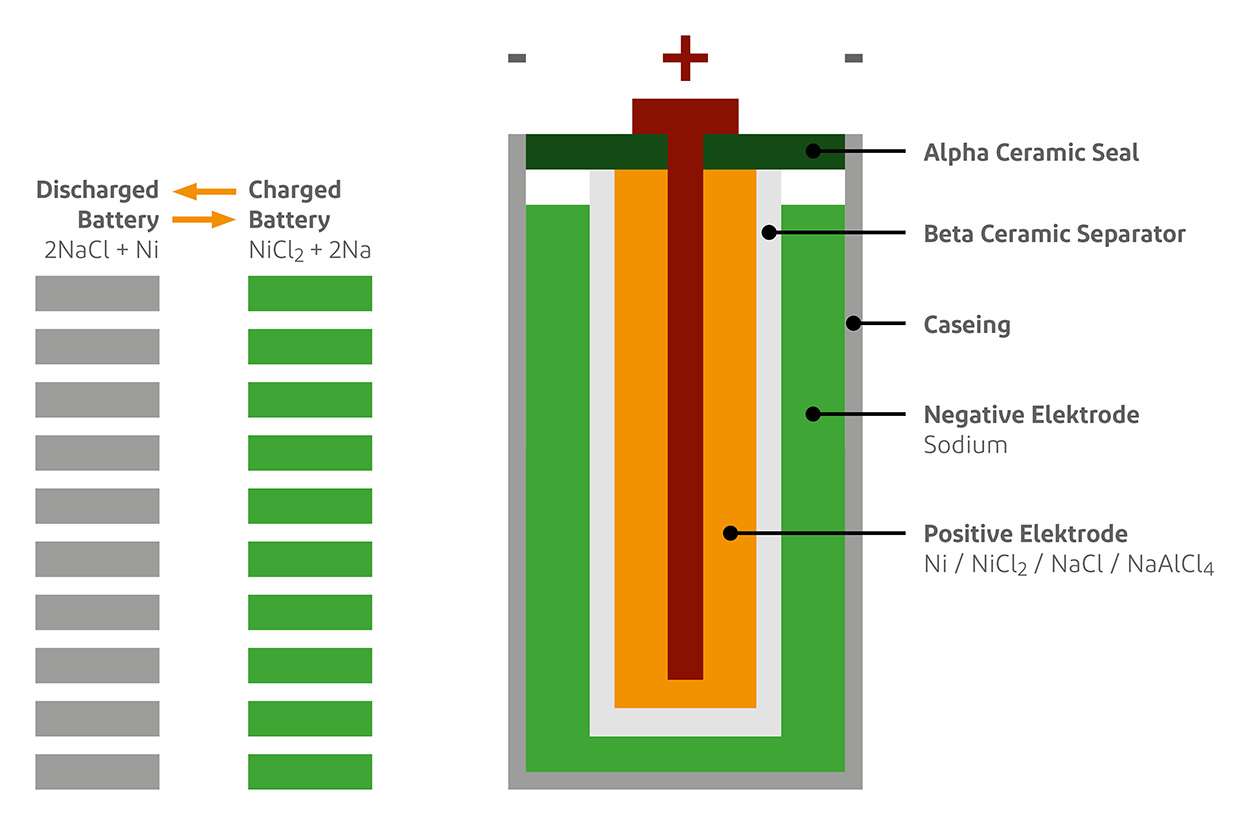
A small NOTE beforehand to avoid confusion. The salt battery is NOT a salt water battery. It is a sodium-nickel battery. More about this below in the chemistry.
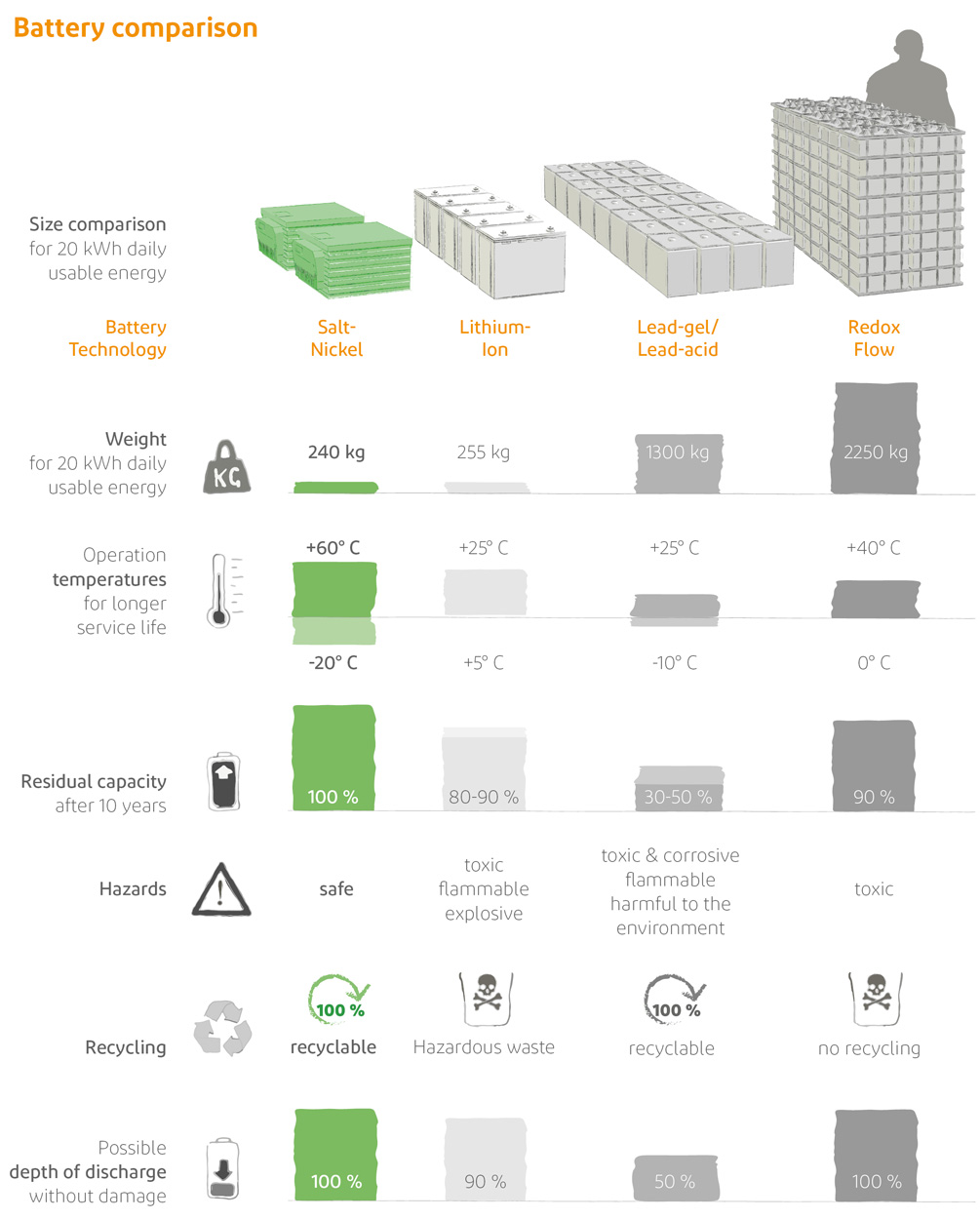
The salt battery compares favourably with other batteries!
In terms of size and weight, the salt battery has approximately the same energy density as lithium-ion batteries. If we look at the temperature range, the salt battery is far ahead of all other battery types. With a temperature range from +60°C to -20°C, it can be operated under hot conditions, such as in Africa, as well as under very cold conditions, such as outside in winter. The remaining capacity after 10 years is still 100 %. Battery death does not happen abruptly, as it does with lead or lithium-ion, but very slowly. If a cell fails, it is bypassed and only the storage capacity is reduced by the small amount of this cell. Otherwise, the battery continues to function as before. When this battery death begins after about 15 years, a new battery can be added at any time. The old battery can continue to be used until its storage capacity has completely disappeared. The battery does not need a discharge buffer, i.e. it can be completely discharged without suffering any damage. It can easily be allowed to cool down, shut down and then be powered up again months later without any problems. Unlike lead-acid and lithium batteries, the salt battery can neither burn nor explode. It requires no ventilation, no air conditioning, no temperature control and no special fire protection or fire warning devices. The salt battery is absolutely safe. It is also not toxic, corrosive or harmful to the environment. It is the salt in the soup of battery storage.
In summary, the salt battery is extremely safe, durable and sustainable. Details on the different salt battery storage systems can be found in the respective product descriptions.
The raw materials - available in abundance worldwide!
The salt batteries used by innovenergy® do not contain any questionable and environmentally harmful substances. All raw materials are abundantly available worldwide. In the search for the right suppliers, great attention was paid to high quality, clean raw material extraction and the shortest possible transport routes. This sustainable approach is also reflected in the fact that the salt battery is manufactured 100% in Switzerland, according to the highest Swiss environmental and labour standards.
32 % Salt
Sodium chloride (NaCL), or common salt, is a vital element. Cassidor said: "We can do without gold, but not without salt." It is available everywhere, including in Switzerland. innovenergy® uses only the high-purity salt from Swiss saltworks. This guarantees short transport routes with lower CO2 emissions.
22 % Iron
The raw material iron (Fe) is mined and processed in very many countries. It is a material that we encounter everywhere in our daily lives. The good thing about it is that no country is so dominant with its mining that it holds a monopoly. innovenergy® sources its steel within Europe.
22 % Nickel
Nickel (Ni) is already a rarer raw material than salt and iron. But nickel is also extracted in a well-distributed manner worldwide. Nickel is an alloying metal that is mostly used in steel refining. Nickel makes steel corrosion-resistant and increases its hardness and toughness. The nickel of innovenergy® comes from Europe.
20 % Ceramics
In technical language, the generic term "ceramic bodies" is a designation for a variety of inorganic non-metallic materials. Colloquially, ceramic is nothing more than very fine clay, of which there are many different varieties all over the world. The high-quality beta-ceramics from Germany and Switzerland are sodium-ion conductive.
A closed cycle - all raw materials are 100% recycled!
When it comes to the term sustainability, you can take a closer look, because it is not only recycling that is important, but also how raw materials are available and mined.
The individual cells as well as the entire salt battery consist of materials that can be recycled after 10 years of use in stationary electricity storage. The recycling of the salt battery has been standardised and industrialised for 15 years. The metals are melted down and returned to the metal industry. Your pan on the cooker could have been a salt battery in the past. The salt and ceramics are further processed in road construction. Thus the material cycle of the salt battery is almost perfectly solved.

Always stay up to date
To make sure you don't miss anything, subscribe to our sporadic newsletter with exciting articles about ecological battery storage and the latest information about our systems.
No, we don't pester you incessantly. The development of good products is closer to us than writing. We just want to share something interesting with you from time to time. Or inform you about system updates.
The relationship drama of Mr. Nickel, Mr. Sodium and Mrs. Chlorine
Take a salt battery: a molten salt (NaAlCl4) at 250 degrees. Mr. Nickel and the dream couple Mr. Sodium and Mrs. Chlorine, also known as common salt by their surnames, hang around in it.
When the sun appears above the horizon every morning, the daily marital tragedy of Mr. Sodium and Mrs. Chlor begins. The charging current of the sun heats up the couple, they quarrel and are separated. Mr. Sodium is suddenly alone, he is missing Mrs. Chlor, an electron. Mrs. Chlor also feels terribly lonely now, looks for new bonding opportunities and meets Mr. Nickel, who has had his eye on the attractive Mrs. Chlor for a long time. As eager as Mrs. Chlor is to form a bond, she unceremoniously marries Mr. Nickel and they form a happy nickel-chloride salt.
And Mr. Sodium is disgruntled and emigrates. Instinctively, he is drawn to a land behind the ceramic electrolytes (beta ceramic separator) and there he finds what he is missing. In this land of milk and honey behind the wall, electrons fall like manna from heaven. Two sodium buddies join forces and treat themselves to two electrons. How heavenly the fusion into pure metallic sodium feels. They are in their element, completely themselves.
When night falls, the great yearning begins. The nimble electrons are fleeting and simply abandon their sodium masters (the battery is discharged), the sodium society breaks apart. And each individual remembers the warm affection of Mrs. Chlorine. Where is she? Behind the separator, perhaps? Homesickness drives her back to her own country.
Mrs. Chlor has also had enough of Mr. Nickel, the boring fellow. And there she sees him, her beloved husband Natrium, returning home repentant. What joy, what a celebration when the two reunite. There is a real release of energy! But all this is exhausting, the whole company gets tired and everyone falls asleep or dozes off. Until the sun sends its merciless rays again the next day and the marriage drama begins anew. Life is a cycle.
The world tour of the salt battery
The salt battery was invented in South Africa and finalised into a product in a joint venture between Anglo-American (money) and AEG (spirit) in Germany.
Batteries are direct current and direct current is in the DNA of the German AEG. Why? Quite simple: AEG was still called "Deutsche Edison-Gesellschaft für angewandte Elektrizität" when it was founded in 1883. Edison was the 4-star general of direct current in the "electricity war" between him and Westinghouse, who represented alternating current. And so AEG took up the salt battery, even when it had long since defected to the alternating current camp. AEG, which long ago also manufactured trams, electric locomotives and, in a subsidiary, even motor vehicles, was bought up by Daimler-Benz AG in 1985.
In the course of the turn of the millennium, Daimler was then infected again by the direct current virus and developed battery and fuel cell vehicles. At that time, a saline battery was installed in the A-Class Mercedes. At that time, General Motors (EV1 with nickel-metal hydride battery), Daimler (A-Class with saline battery) and Ford (Think with saline battery) were the worldwide pioneers of electric mobility. Back then!
Now we know that Emperor Petroleum, the world ruler of the energy industry, has again cleverly distracted the automobile companies from the trials and tribulations of renewable electric mobility for a time with gentle force. Daimler was no exception. The salt battery factory was sold and ended up in Stabio, Switzerland, under the reign of FIAMM, where it still is today. It enjoys excellent health and produces around 750,000 cells per year.
And for the old hands who have been around the battery business for a while: innovenergy® uses the then so-called ZEBRA battery from Daimler.
This mature and proven technology has been successfully used in the telecommunications industry for over 20 years and is now finding its way into the world of renewable energy in private households as well as commercial battery storage uses, where it is much better off than in its original automotive application.